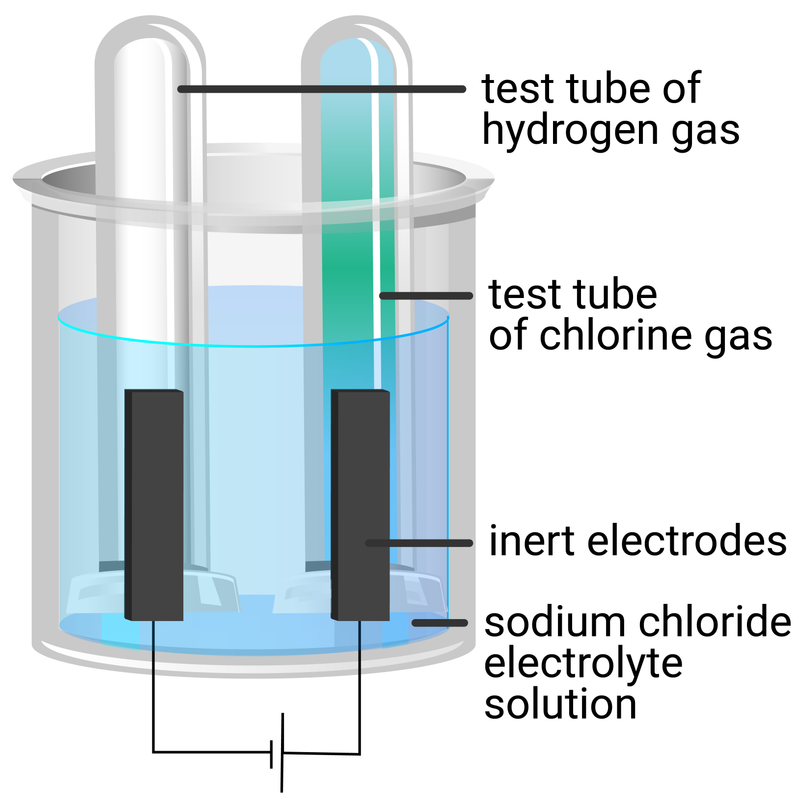
Electrolysis Of Table Salt . Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Web electrolysis of molten sodium chloride. Web describe the process of electrolysis; We start with a salt solution, for example, potassium. Compare the operation of electrolytic cells with that of galvanic cells; Web a salt like $\ce{na2so4}$ is essential in electrolysis. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Let’s take a closer look. Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water.
from revisechemistry.uk
Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Web electrolysis of molten sodium chloride. Compare the operation of electrolytic cells with that of galvanic cells; Web describe the process of electrolysis; Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Web a salt like $\ce{na2so4}$ is essential in electrolysis. Let’s take a closer look. We start with a salt solution, for example, potassium. Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas.
Electrolysis OCR GCSE PAG 2 revisechemistry.uk
Electrolysis Of Table Salt Compare the operation of electrolytic cells with that of galvanic cells; Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Web describe the process of electrolysis; Web a salt like $\ce{na2so4}$ is essential in electrolysis. Let’s take a closer look. We start with a salt solution, for example, potassium. Web electrolysis of molten sodium chloride. At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. Compare the operation of electrolytic cells with that of galvanic cells; Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although.
From www.slideserve.com
PPT Electrolysis of salt 3 PowerPoint Presentation, free download Electrolysis Of Table Salt Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Web electrolysis of molten sodium chloride. Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water. We start with a salt solution, for example, potassium. Web 2h 2 o (l) + 2nacl. Electrolysis Of Table Salt.
From courses.lumenlearning.com
Electrolysis Chemistry Atoms First Electrolysis Of Table Salt Compare the operation of electrolytic cells with that of galvanic cells; Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Web describe the process of electrolysis; At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Let’s take a closer. Electrolysis Of Table Salt.
From revisechemistry.uk
Electrolysis OCR GCSE PAG 2 revisechemistry.uk Electrolysis Of Table Salt Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Compare the operation of electrolytic cells with that of galvanic cells; We start with a salt solution, for example, potassium. Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Let’s take. Electrolysis Of Table Salt.
From www.slideserve.com
PPT Electrolysis of Salt Water PowerPoint Presentation, free download Electrolysis Of Table Salt Web a salt like $\ce{na2so4}$ is essential in electrolysis. Web electrolysis of molten sodium chloride. At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. We start with a salt solution, for example, potassium. 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Web describe the process of electrolysis; Molten (liquid) sodium chloride can be electrolyzed. Electrolysis Of Table Salt.
From wigtonphysics.blogspot.com
wigton physics Electrolysis of an aqueous solution of sodium chloride Electrolysis Of Table Salt Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Compare the operation of electrolytic cells with that of galvanic cells; Web describe the process of electrolysis; Web. Electrolysis Of Table Salt.
From chem.libretexts.org
Electrolysis I Chemistry LibreTexts Electrolysis Of Table Salt Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Web describe the process of electrolysis; Web a salt like $\ce{na2so4}$ is essential in electrolysis. Let’s take a closer look. We start with a salt solution, for example, potassium. Web in this explainer, we will learn how to predict the products of electrolysis of. Electrolysis Of Table Salt.
From patents.google.com
WO2009007691A2 Electrolysis of salt water Google Patents Electrolysis Of Table Salt Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water. Let’s take a closer look. Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Compare. Electrolysis Of Table Salt.
From www.youtube.com
Electrolysis of Salt Solution at Home in Under 1 minute to produce Electrolysis Of Table Salt Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. We start with a salt solution, for example, potassium. Web describe the process of electrolysis; Web electrolysis of molten sodium chloride. At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Web 2h. Electrolysis Of Table Salt.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolysis Of Table Salt At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. Web a salt like $\ce{na2so4}$ is essential in electrolysis. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Compare the operation of electrolytic cells with that of galvanic cells; Web 2h 2 o (l) +. Electrolysis Of Table Salt.
From www.tessshebaylo.com
Electrolysis Of Salt Water Half Equations Tessshebaylo Electrolysis Of Table Salt Let’s take a closer look. Web describe the process of electrolysis; Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water. 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid) sodium chloride. Electrolysis Of Table Salt.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Electrolysis Of Table Salt Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Web in this explainer, we will learn how to predict. Electrolysis Of Table Salt.
From printablebispa6t.z21.web.core.windows.net
Electrochemical Cells Gcse Chemistry Electrolysis Of Table Salt At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. Web electrolysis of molten sodium chloride. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Web 2h 2 o (l) +. Electrolysis Of Table Salt.
From www.savemyexams.com
Electrolysis of Aqueous Solutions (4.1.4) CIE IGCSE Chemistry Electrolysis Of Table Salt Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Web describe the process of electrolysis; Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Compare the operation of electrolytic cells with that of galvanic cells; At high salt concentration, hydrogen and chlorine. Electrolysis Of Table Salt.
From www.thoughtco.com
Chemical Composition of Table Salt Electrolysis Of Table Salt Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water. Web a salt like $\ce{na2so4}$ is essential in electrolysis. At high salt concentration, hydrogen and chlorine is the product of electrolysis with. Electrolysis Of Table Salt.
From mungfali.com
Sodium Chloride Electrolysis Equation Electrolysis Of Table Salt Web a salt like $\ce{na2so4}$ is essential in electrolysis. Web electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Compare the operation of electrolytic cells with that of galvanic cells; 1:14in this video, i'm electrolyzing sodium hydroxide to produce. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal. Electrolysis Of Table Salt.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Electrolysis Of Table Salt Web describe the process of electrolysis; At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium. Web 2h 2 o (l) + 2nacl → h 2 (g) + cl 2 + 2naoh. Web electrolysis of molten sodium chloride. Compare the operation of electrolytic cells with that of galvanic cells; Web a salt like $\ce{na2so4}$ is essential. Electrolysis Of Table Salt.
From partdiagramvarenesch01.z14.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrolysis Of Table Salt Web a salt like $\ce{na2so4}$ is essential in electrolysis. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Web so the electrolysis of salt solutions is the electrical separation of ions dissolved in water. Web electrolysis of molten sodium chloride. Compare the operation of electrolytic cells with that of galvanic cells; Web describe the process. Electrolysis Of Table Salt.
From www.coursehero.com
[Solved] Analyzing the electrolysis of molten salt Suppose a powerful Electrolysis Of Table Salt Web describe the process of electrolysis; Web in this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Web electrolysis of molten sodium chloride. Web a salt like $\ce{na2so4}$ is essential in electrolysis. Compare the operation of electrolytic cells with that of galvanic cells; Molten (liquid) sodium chloride can be. Electrolysis Of Table Salt.